


U.S. Patent Item Authorization K- INNOVATIVE PRODUCT
InnoMyco vaccine
Components
- Mycoplasma hyopneumoniae HID3140
- Recombinant P97 protein derived from Mycoplasma hyopneumoniae
- Mycoplasma hyorhinis HID3224
- IMS1313
Efficacy and effectiveness
- Prevention and Symptom Relief of Swine Mycoplasma Pneumonia
- Provides high safety without any side effects
Usage and Capacity
- 1 injection of 2mL into a pig of 3 weeks or older
- Shake enough vaccine before use
InnoMyco
- The world's first Swine Pneumonia Vaccine by new antigens (U.S. Patent Registration)
- High Efficacy Vaccine for Symptom Relief and Prevention of Swine Mycoplasma Pneumonia
- Acguired K- INNOVATIVE PRODUCT Certification(Korea INSTITUTE OF PLANNING AND EVALUATION FOR TECHNOLOGY)
Product Overview
| Product Name | InnoMyco |
|---|---|
| Time for vaccination | 1 inoculation to pigs over 3 weeks old |
| Vaccination method | Injection once by Intramuscular injection with 2mL of vaccine |
| Efficacy and effectiveness | Prevention of Swine Mycoplasma Pneumonia |
Product Properties
- Effectively defends both Mycoplasma hyopneumoniae (Mhp) and Mycoplasma hyorhinis (Mhr)
- Stimulates strong humeral and cell-mediated immunity
- Recommended for early prevention of infection and relief from symptoms
| Excellent induction of immunity | Economic effects |
|---|---|
|
|
Traditional Mycoplasma vaccine VS InnoMyco vaccine
| existing vaccine products |
|---|
| Weakness in antibody formation (Antibodies could not be measured in commercial diagnostic kits) |
| Not contained (Inability to prevent early infections) |
| Not contained |
| Weakness |
| Weakness |
| INNOVAC’s vaccine |
|---|
| Excellent antibody formation ability against Mhp |
| Contains Mhr-P97 attachment factors |
| Contains the new pathogen Mhr of pneumonia |
| Excellent in somatic immunity |
| Excellent in cell-mediated immunity |

Vaccine efficacy and effect (evaluation result of the Innomyco vaccine in test farm)
이노마이코 접종군
대조군
Significant reduction in lung lesions
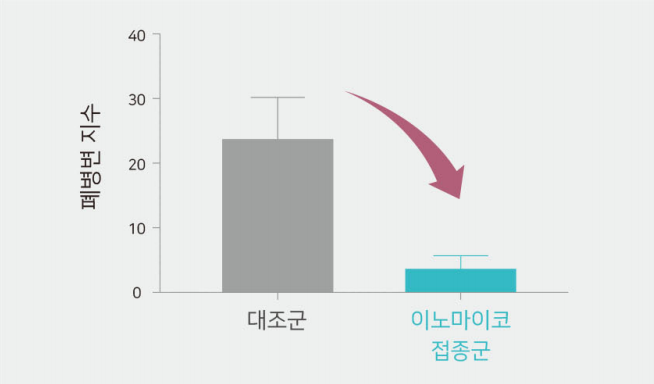
- 83% reduction in lung lesions after vaccination
- Identifying outstanding preventive effects on mycoplasma pneumonia
Excellent cell mediated immunity
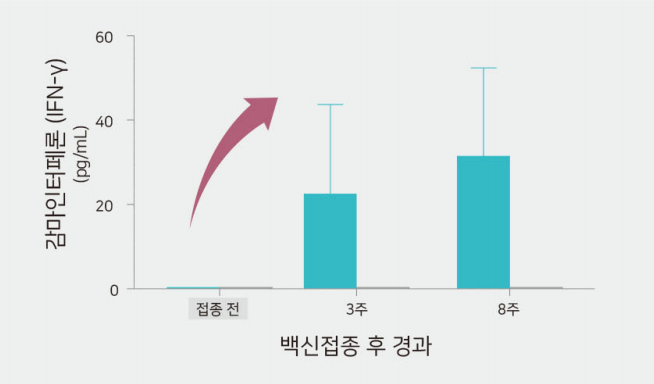
- Identifying excellent production of gamma-interferon secretion immune cell on Mhp
Increase antibody-mediated immunity
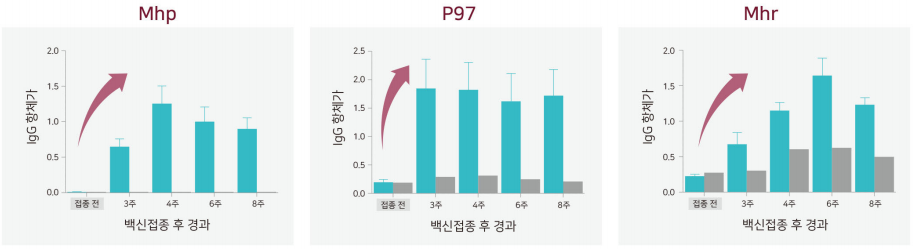
- Identifying excellent antibody formation for major pathogenic antigens
- Vaccination effect can be checked using existing serum test (In case of Mhp antigen, it can be tested with IDEXX kit)
